-
Type:
Hosp Inpt eCQMs - Hospital Inpatient eCQMs
-
Resolution: Answered
-
Priority:
High
-
Component/s: ValueSet
-
Lyn S. Gurney, RN, BSBL, CPHQ
-
(601) 249-1825
-
Southwest Mississippi Regional Medical Center
-
-
CMS0506v7
-
[Suggested solution text preserved from the Solution field after the response was posted:
I've included a screenshot of ValueSet CMS provided for this measure to EPIC (See attached ValueSetCMSprovided.jpg)
Looking at this, you will see the Schedule V opioid controlled substance included.
Furthermore, we would like an answer provided to us with how far, since mandatory reporting began in 2023, we can go back and correct our data for resubmission to CMS pertaining to this metric.
Epic requires an official statement from CMS acknowledging the error and to issue a new, corrected "value set" sans the identified error. Also, to audit the set for any other Schedule V opioid controlled substances and remove those as well.]
To Whom It May Concern:
Apparently, the value set provided by CMS to Epic for the metric CMS506v7 and/or prior versions had "codeine phosphate 2 MG/ML / guaifenesin 20 MG/ML Oral Solution" grouped with the category of Schedule II - IV opioid controlled substances.
Epic requires an official statement from CMS acknowledging the error and to issue a new, corrected "value set" sans the identified error.
If you are unable to directly address this matter, please feel free to forward this message to those who are able to address this concern.
Furthermore, we would like an answer provided to us with how far, since mandatory reporting began in 2023, we can go back and correct our data for resubmission to CMS pertaining to this metric.
From the following link, a listing for "Codeine preparations - 200 mg/(100 ml or 100 mg)" can be found:
https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf
The following is a snippet found after navigating to the aforementioned link and searching for, "Codeine preparations - 200 mg/(100 ml or 100 mg."
An oral solution containing 2 mg/mL of codeine phosphate and 20 mg/mL of guaifenesin is a federal Schedule V controlled substance. 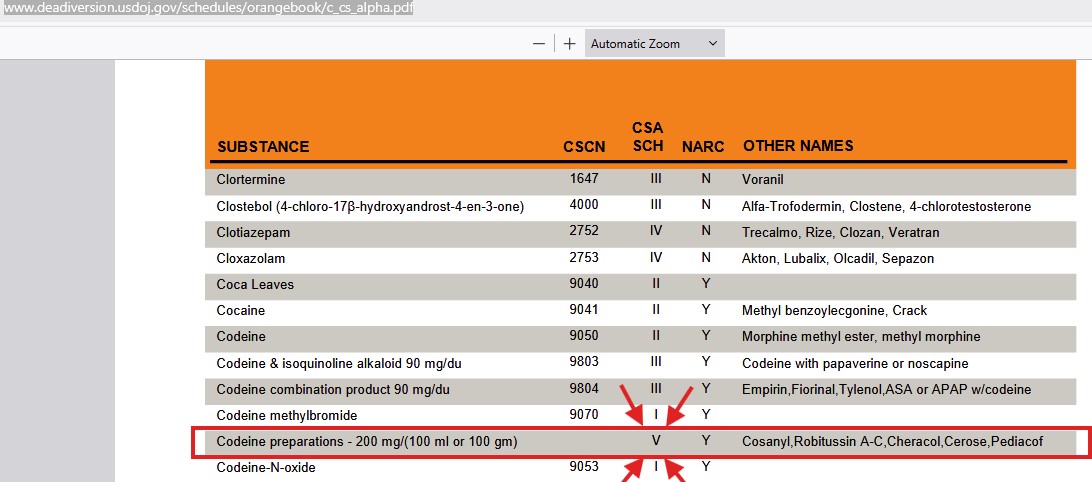
- Is blocked by
-
EKI-45 CMS506v7/CMS506v8
-
- To Do
-