Foundational Documents |
| Document | Description | Download | View |
|---|
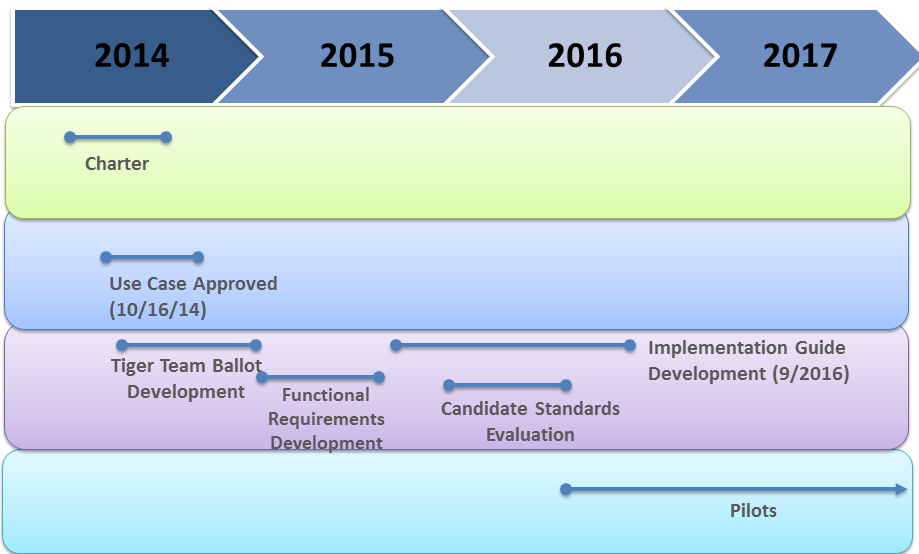
| Data Provenance Charter Document | The Charter document is the first document to propose the need for tracking Data Provenance across Health IT. It describes the challenges, scope, stakeholders for this initiative as well as defining the value of the project and the first potential set of data elements needed to ensure security across a medical record's lifecycle. | Download Document | View PDF |
| Data Provenance Functional Requirements Document | The Functional Requirements document goes in depth defining all of the data elements needed for a successful pilot demonstration of provenance. | Download Document | View PDF |
| Use Case | The Use Case document reviews the scenarios in which Data Provenance can be applied. | Download Document | View PDF |
DPROV General Reference Materials |
| Document | Description | Download |
|---|
| Data Provenance Initiative Launch | The first meeting for the Data Provenance Initiative held on April 10, 2014. Click here to view the recording. | Download PowerPoint |
| Data Provenance Executive Summary | The Data Provenance Executive Summary is a high level overview made to attract pilots, describing the need for Data Provenance and showcasing the initiative's Use Case set | Download PDF |
| Data Provenance Glossary | This page provides a list of important terms to the DPROV Initiative | Visit Wikipage |
| DPROV HL7 Artifacts | This links to the HL7 Data Provenance Wiki page, providing all HL7 artifacts connected to the DPROV Initiative | Visit Wikipage |
| Health IT Standards Committee: Recommendations to the National Coordinator for Health IT | This page describes the Standards recommendations for ONC | Visit Website |
| Implementing Interoperable Provenance in Biomedical Research | A document by Vasa Curcin, et al describing how to implement provenance in biomedical domains | Download Document |
| ONC-SI: Data Provenance - FHIR Provenance Resource | FHIR DSTU Release 1.1 Provenance Resource Presentation from January 16, 2015 | Download Document |
HL7 Implementation Guide for CDA® Release 2: Data Provenance, Release 1 – US Realm | This IG provides guidance to any CDA R2 implementer on the use of CDA templates to represent the data provenance as well specifying reusable models as building blocks for use in other information exchange standards | Visit HL7 Standards Website |
| Provenance-FHIR 0.4.0 | PDF of the FHIR Provenance Resource | Download PDF |
| ONC-SI: Provenance Lifecycle Event Standards | System Functional and Lifecycle Model Presentations | Download Document |
| Trusted End-to-End Information Flows | ISO 21089 Presentation regarding Trusted End-to-End Information Flows | Download Document |
| ONC-SI: Provenance-Record Lifecycle | EHR Functional Model Presentation from June 26, 2014 | Download Document |
| ONC-SI: Provenance-Record Lifecycle Events Across SI Initiatives | EHR Record Lifecycle Events and Data Provenance Across ONC initiatives from July 1, 2014 | Download Document |
| CentriHealth Comments Actors-Roles-Accountability | CentriHealth comments on Actors, Roles, and Accountability from August 10, 2014 | Download Document |
| CentriHealth Comments Assumptions-Pre-Post-Conditions | CentriHealth comments on Assumptions and Pre/Post-Conditions from August 10, 2014 | Download Document |
| CentriHealth Comments User Story Examples | CentriHealth comments on User Story Examples from August 10, 2014 | Download Document |
| DPROV Project Summary | The Summary is a high-level overview of the DPROV initiative, covering all aspects from inception, to execution and piloting. | Download
Document |