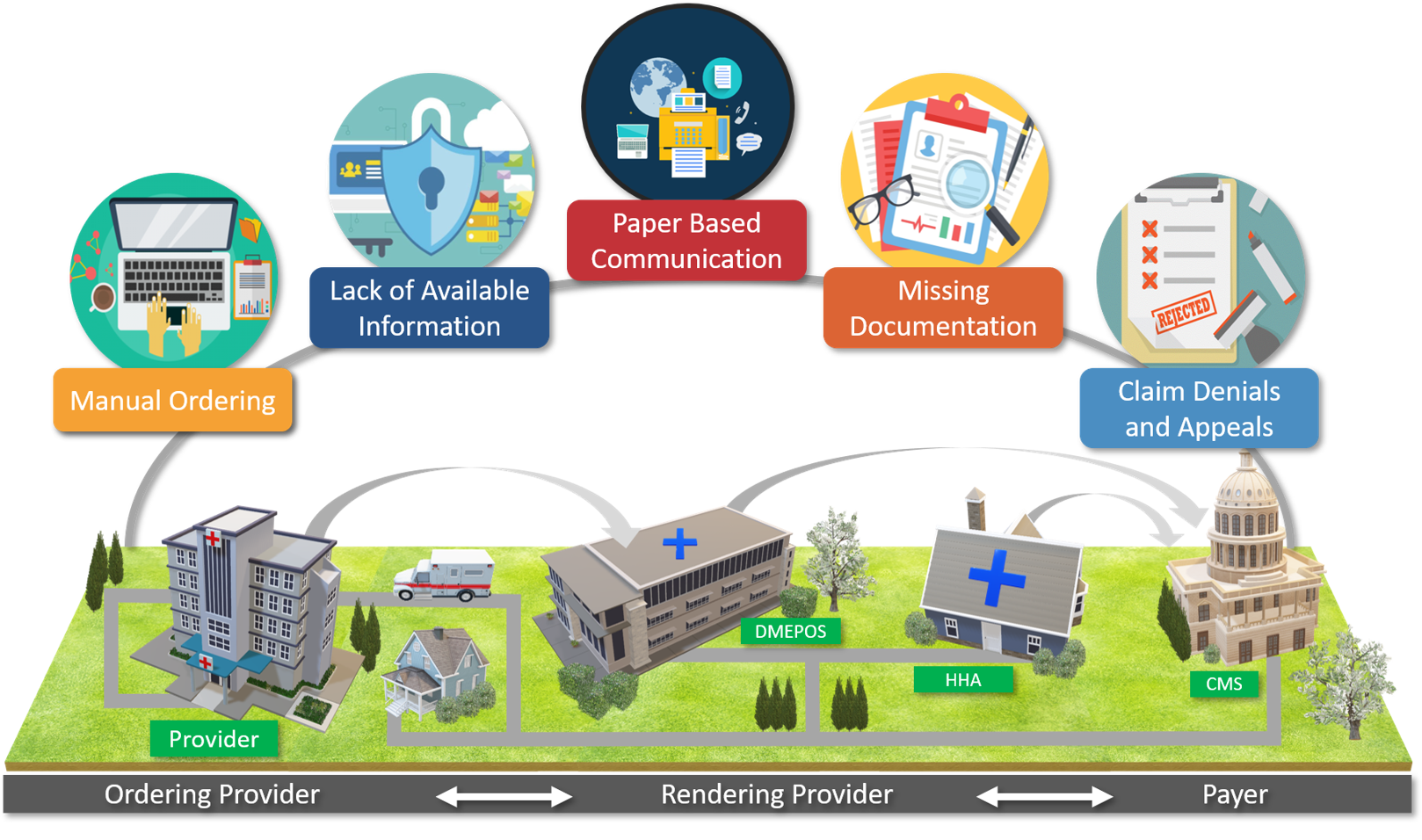
The EMDI Implemenation Guide is intended for healthcare providers and stakeholders who are interested in participating in the EMDI program. These organizations should have the capability to build the architecture and the supporting infrastructure necessary for interoperability. The primary intended audiences are healthcare providers, such as hospitals, physicians, Home Health Agency (HHA) services, Durable Medical Equipment, Prosthetic, Orthotic, & Supplies (DMEPOS), labs, comprehensive primary care networks such as Comprehensive Primary Care Plus (CPC+), and virtual physician networks. Other audiences include: - Document transfer vendors, such as Health Information Handlers (HIH), Heath Information Service providers (HISP), and clearinghouses.
- Interface Vendors, such as Electronic Health Records (EHR) and document management system vendors.
- Other payers, such as Medicaid State Agencies and commercial payers.
- IT vendors involved in facilitating the physician’s digital signature on medical record documents.
- Suppliers and ambulance providers
|